

Fourteen-karat gold-copper alloy is nearly identical in color to certain bronze alloys, and both may be used to produce police and other badges. Alloys containing palladium or nickel are also important in commercial jewelry as these produce white gold alloys. Similar effects impart a golden hue to metallic caesium.Ĭommon colored gold alloys include the distinctive eighteen-karat rose gold created by the addition of copper. This color is determined by the frequency of plasma oscillations among the metal's valence electrons, in the ultraviolet range for most metals but in the visible range for gold due to relativistic effects affecting the orbitals around gold atoms. Whereas most metals are gray or silvery white, gold is slightly reddish-yellow. By comparison, the density of lead is 11.34 g/cm 3, and that of the densest element, osmium, is 22.588 ☐.015 g/cm 3. Gold has a density of 19.3 g/cm 3, almost identical to that of tungsten at 19.25 g/cm 3 as such, tungsten has been used in counterfeiting of gold bars, such as by plating a tungsten bar with gold. Gold is a good conductor of heat and electricity. Such semi-transparent sheets also strongly reflect infrared light, making them useful as infrared (radiant heat) shields in visors of heat-resistant suits, and in sun-visors for spacesuits. The transmitted light appears greenish-blue, because gold strongly reflects yellow and red. Gold leaf can be beaten thin enough to become semi-transparent. A single gram of gold can be beaten into a sheet of 1 square metre (11 sq ft), and an avoirdupois ounce into 300 square feet (28 m 2). Such nanowires distort via formation, reorientation and migration of dislocations and crystal twins without noticeable hardening. It can be drawn into a wire of single-atom width, and then stretched considerably before it breaks. Gold is the most malleable of all metals. Certain gold salts are still used as anti-inflammatories in medicine.Ī gold nugget of 5 mm (0.20 in) in size can be hammered into a gold foil of about 0.5 m 2 (5.4 sq ft) in area. Gold is also used in infrared shielding, production of colored glass, gold leafing, and tooth restoration.
Gold's high malleability, ductility, resistance to corrosion and most other chemical reactions, and conductivity of electricity have led to its continued use in corrosion-resistant electrical connectors in all types of computerized devices (its chief industrial use). The world consumption of new gold produced is about 50% in jewelry, 40% in investments and 10% in industry.

This is equal to a cube with each side measuring roughly 21.7 meters (71 ft). A total of around 201,296 tonnes of gold exists above ground, as of 2020. In 2020, the world's largest gold producer was China, followed by Russia and Australia. Gold coins ceased to be minted as a circulating currency in the 1930s, and the world gold standard was abandoned for a fiat currency system after the Nixon shock measures of 1971. In the past, a gold standard was often implemented as a monetary policy. Gold also dissolves in mercury, forming amalgam alloys, and as the gold acts simply as a solute, this is not a chemical reaction.Ī relatively rare element, gold is a precious metal that has been used for coinage, jewelry, and other arts throughout recorded history. Gold dissolves in alkaline solutions of cyanide, which are used in mining and electroplating. Gold is insoluble in nitric acid alone, which dissolves silver and base metals, a property long used to refine gold and confirm the presence of gold in metallic substances, giving rise to the term ' acid test'. Gold is resistant to most acids, though it does dissolve in aqua regia (a mixture of nitric acid and hydrochloric acid), forming a soluble tetrachloroaurate anion. Less commonly, it occurs in minerals as gold compounds, often with tellurium ( gold tellurides).
#Information about gold element series
It occurs in a solid solution series with the native element silver (as in electrum), naturally alloyed with other metals like copper and palladium, and mineral inclusions such as within pyrite.
#Information about gold element free
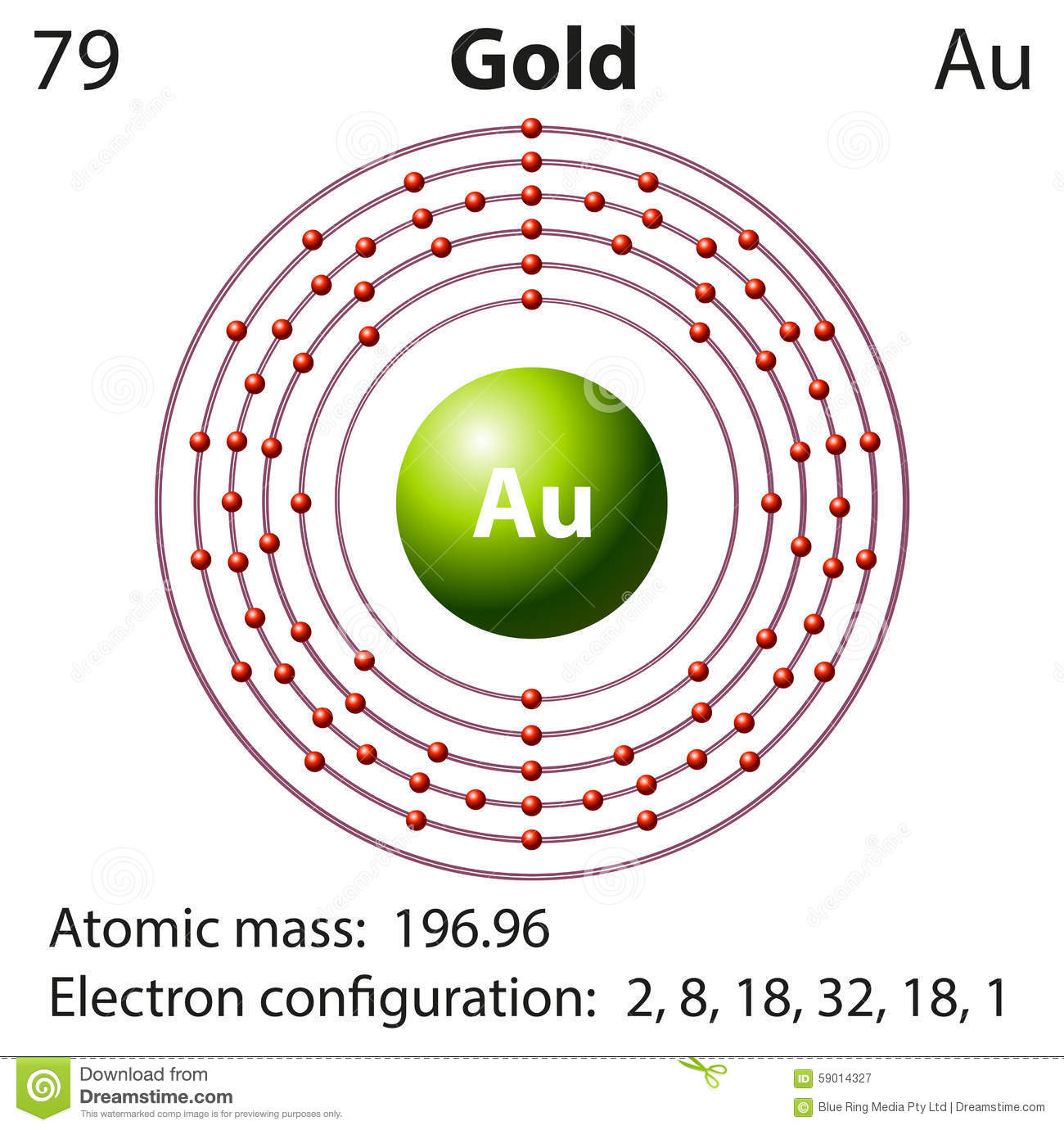
Gold often occurs in free elemental ( native state), as nuggets or grains, in rocks, veins, and alluvial deposits. It is one of the least reactive chemical elements and is solid under standard conditions. Chemically, gold is a transition metal and a group 11 element. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile metal in pure form. This makes it one of the higher–atomic-number elements that occur naturally. Gold is a chemical element with the symbol Au (from Latin aurum 'gold') and atomic number 79.


 0 kommentar(er)
0 kommentar(er)
